- Oh hey. Trout season opened yesterday. Tomorrow, two days of stalking brown trout on the credit… homemade flies and super light tippet. #
Sugar, part 3
From a culinary perspective, sugar is a very important ingredient so understanding how it reacts while being heated, melted, dissolved, etc is very crucial. One of the most integral parts of this is an understanding of what happens in a simple sugar and water solution. In this case, the sugar molecules have a tendency to bond to each other in orderly arrays and form dense solid masses (crystals). When sugar crystals are dissolved in water, H2O molecules have a tendency to overcome this bond between sugar molecules and form their own bonds. If the sugar molecules become too crowded with the water molecules, they begin pairing off with each other and form crystals once again.
One way to change the amount of sugar and water bonds in a simple syrup (sugar + water) is through an increase (or decrease) in temperature. As a sugar solution boils, water molecules evaporate from the liquid while the sugar molecules remain behind changing the concentration of the syrup (also its boiling point) and the amount of bonds present. Such a phenomenon is very important in the making of candies, meringues and various other desserts.
Remember, as you cook your sugar syrup, most of the heat goes into the work of evaporating the water molecules and less into actually rising the temperature of the liquid as a whole, so the temperature reading will rise only gradually. However, as the sugar concentration passes 80%, there’s very little water left to evaporate so the temperature of the syrup will begin rising very rapidly. A candy (or digital) thermometer as crucial when making any recipes including a sugar syrup.
A cold water test is another way of getting a syrup behaviour reading without the use of a thermometer. This test requires a small sample of the syrup to be cooled quickly (dropped in cold water) and then noting its behaviour. Thin syrups simply form a thread in the air, more concentrated solutions form a malleable ball while others make very hard brittle threads.
Here’s a general list of temperatures, the cold water test result and what confections they’re predominantly used for:
102-113C – malleable threads – used in syrups and preserves
113-116C – soft ball stage – used in fondants and fudge
118-121C – firm ball stage -used for caramels
121-130C – hard ball stage – used in marshmallows and nougat
132-143C- soft crack stage (very brittle) – used for taffy
149-154C – hard crack stage – used in butterscotch and brittle
160-168C – beginning to turn colour, cold water test no longer applies – used in hard candies and toffee
180-182C – has a deep golden brown colour – used in spun sugar, medium caramel
188-190C – a reddish brown colour – used for dark caramel
Sugar, part 2
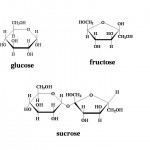
There are several different types of sugars, all with various qualities and sweetness levels. Different sugars give different impressions of sweetness with sucrose being the longest to be detected on the tongue and the most lingering of the bunch. By comparison, fructose registers quickly and strongly but it also fades very quickly. But before we talk more about the complexities of sugars, lets define all of of them:
Glucose (aka Dextrose)
It is a simple sugar, the most common sugar from which living cells directly extract chemical energy. It can be found in many fruits and in honey but always in a mixture with other sugars. It is the building block from which starch chains are constructed.
Glucose is less sweet, less soluble in water and produces a thinner solution than table sugar. It also melts and begins to caramelize at around 300F/150C.
Fructose (aka Levulose)
It has the exact same chemical formula as glucose with the atoms being arranged in a different structure. Like glucose, it is found in fruits and honey.
Fructose is the sweetest of the common sugars, the most soluble in water and it absorbs and retains water the most effectively. However, our bodies metabolise fructose more slowly than glucose and sucrose so it causes a slower rise in blood glucose levels. It melts and caramelizes at around 220F/105C.
Sucrose (aka table sugar)
It is a composite molecule made from one molecule each of glucose and fructose. Green plants produce this compound in the process of photosynthesis and of all the common sugars it has the most useful combination of properties. It is the second sweetest but it is the only one have a pleasant taste even at very high concentrations.
Sucrose is also the second most soluble sugar and it produces the greatest viscosity in a water solution. It begins to melt around 320F/160C and caramelizes at around 340F/170C.
When heated in the presence of some acid or around certain enzymes, sucrose breaks apart into two subsugars. Breaking sucrose into glucose and fructose is often referred to as “inversion” and the resulting mixture is referred to as an invert sugar. Generally this syrup contains 75% glucose and fructose and 25% sucrose; and only exists in liquid form because the fructose component never fully crystallizes.
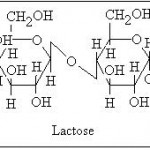
Lactose
It is the sugar found in milk products and a composite of two simple sugars, glucose and galactose. It is much less sweet than table sugar and used predominantly for sugar alcohols and its physical bulk than for its sweetness.
Sugar week, Part 1
I thought it would be neat to explore a new topic every week and learn something new, so this week we’re going to talk about sugar. We’ll get into history, uses, the nature of sugars and its varieties.
Sugar is the generalised term used for a class of sweet flavoured substances composed of carbon, hydrogen and oxygen (aka carbohydrates). It is formed naturally in the leaves of numerous plants but concentrated mainly in their roots, stems, or fruits. It can be extracted from maple trees, palm trees (toddy, coconut and date palms), sorghum (a type of sugar grain) but commercially comes mostly from sugar canes and sugar beets.
Before there was sugar, there was honey. Paintings in the Spider Cave of Valencia depict humans collecting honey approximately 10, 000 years ago, with the domestication of bees going back 4, 000 years (Egyptian hieroglyphs showing clay hives). Sometime around 500 BC, people in India developed the technology of making unrefined, “raw” sugar by pressing out the cane juice and boiling it down to a dark mass of syrup-coated crystals (called “gur” or “jaggery”). By 350 BC, this sweet solid was being combined with wheat, barley, rice flours and sesame seeds to make all sorts of confections. Around 6th century AD sugar making technologies were carried westward towards the Persian Gulf. It was from here that Europe first encountered sugar during their explorations and Venice became the hub of sugar trade from the Arab countries.
The 18th century showed a boom in European sugar consumption due mostly to the colonial rule in the West Indies and the enslavement of millions of Africans. After years of revolt, the very countries that had brought the slaves out to the plantations began outlawing slavery and therefore a new method of producing the sweet stuff needed to be found. In 1747, a Prussian chemist, Andreas Marggraf used brandy to extract sugar crystals similar to those of the sugar cane from a common European root vegetable, the white beet. It was only during Napoleon’s time in 1812 that the first sugar beet factory was designed.
In the present, 30% of the world’s sugar production comes from beets with Russia, Germany and the US being its top producers. Although the Carribean still play a role in the production of cane sugar, India and Brazil have taken the leading roles in that market.
What went down this week.
- An interesting local food video from Hellmans mayo and the creative folks at Ogilvy. Bit of an eye opener. http://t.co/rxjerHw4 #
- Farms Without Soil Take Root In The Middle East http://t.co/6o06t0hU #
- Amazing old school butchery video from 1945. Beautiful beef, mad skills and banging soundtrack. http://t.co/T1MB9ORc #
- Monsanto, A Potential Contributor To Colony Collapse Disorder, Buys Bee Research Firm http://t.co/3MCgwRmB #
- @FannyChadwicks, thanks for a great meal. Great atmosphere. Loved the terrine and fried chicken.. Glad you're in the neighborhood. #
- A Silicon Valley-Style Incubator For Local Food http://t.co/AeLXQMj9 #
- Media, Consumers Take BSE In Stride http://t.co/r6bEGMPq #
- Lose 20 lbs. of Fat in 30 Days http://t.co/NZ538LGN #
- Hacked account again. Working on it. Sorry for the garbage posts. What a pain.Thanks a bunch to who ever it is for the fun and aggravation. #
Oh hey. Trout season opened ye…
Oh hey. Trout season opened yesterday. Tomorrow, two days of stalking brown trout on the credit… homemade flies and super light tippet.
Hacked account again. Working …
Hacked account again. Working on it. Sorry for the garbage posts. What a pain.Thanks a bunch to who ever it is for the fun and aggravation.
Media, Consumers Take BSE In S…
Media, Consumers Take BSE In Stride http://t.co/r6bEGMPq
A Silicon Valley-Style Incubat…
A Silicon Valley-Style Incubator For Local Food http://t.co/AeLXQMj9
@FannyChadwicks, thanks for a …
@FannyChadwicks, thanks for a great meal. Great atmosphere. Loved the terrine and fried chicken.. Glad you’re in the neighborhood.
Monsanto, A Potential Contribu…
Monsanto, A Potential Contributor To Colony Collapse Disorder, Buys Bee Research Firm http://t.co/3MCgwRmB
Amazing old school butchery vi…
Amazing old school butchery video from 1945. Beautiful beef, mad skills and banging soundtrack. http://t.co/T1MB9ORc
The Beauty of Old School Butchery
I came across this video on Youtube. Its from the U.S. National Archive and was produced in 1945. The butchery skills are amazing as is the music and the narration. Note the caliber of the cow at the begingin of the video and the color and marbling of the meat. This movie was made before factory farming came into its own. Enjoy.
Part 1
Part 2
Farms Without Soil Take Root I…
Farms Without Soil Take Root In The Middle East http://t.co/6o06t0hU
An interesting local food vide…
An interesting local food video from Hellmans mayo and the creative folks at Ogilvy. Bit of an eye opener. http://t.co/rxjerHw4